Blog
Corrosion of steels - Part I
The introduction to this issue will be somewhat longer, although I will simplify and shorten it as much as possible. The reason for this is that anyone who needs to solve this problem needs to keep in mind everything that will be presented here, all at the same time, as one connected and related package.
Above all, it is necessary to realize that steel corrosion is not only a physical and chemical process, but primarily an electro-chemical process. The word "electric" plays a vital role in corrosion.
Iron (Fe) is "missing" 2 electrons in its atom, so it likes to take them from a number of "donors". The "donors" may not only be oxygen (O 2 ), but many other elements. Or, conversely, iron itself can donate its electrons to another element. But as soon as a "hole" in the electron shell of iron is filled or enlarged, this "hole" then jumps further along the Fe metal lattice. For steels, it is usual for it to travel about 30 cm away from the place of its origin.
But steel is not just iron, but a mixture of dozens of elements. Some are alloying, changing the physical and chemical properties of steels in the desired direction, some are present thanks to the entire production process. And most of them have a great influence on the corrosion properties of steels. A number of them, in contact with iron, create a micro galvanic cell, i.e. the germ of corrosion.
But micro galvanic cells can also be created in other ways. Contact with the machining fluid, impurities in the air, the presence of certain ions in washing fluids, etc.
Then at the same time we have the problem of creating galvanic cells. (For example, roxors freshly embedded in concrete create a voltage of 1.2-1.4 V.) But these galvanic cells also arise if one machined surface comes into contact with another (contact, crevice corrosion), or if the product has an unevenly machined surface (different roughness on the same product), especially if different metals or different batches of the same grade of steel come into contact, but the overall shape of the product also plays an important role.
And since it is an electro-chemical process, corrosion rates are several times higher if they take place in an electrically conductive environment. They can also take place in an electrically non-conductive environment, but the speeds in such cases tend to be low. The most common electrically conductive medium is water. And not only in its liquid form, but also in vapor. The dependence of the corrosion rate on the relative air humidity is shown in the following graph.
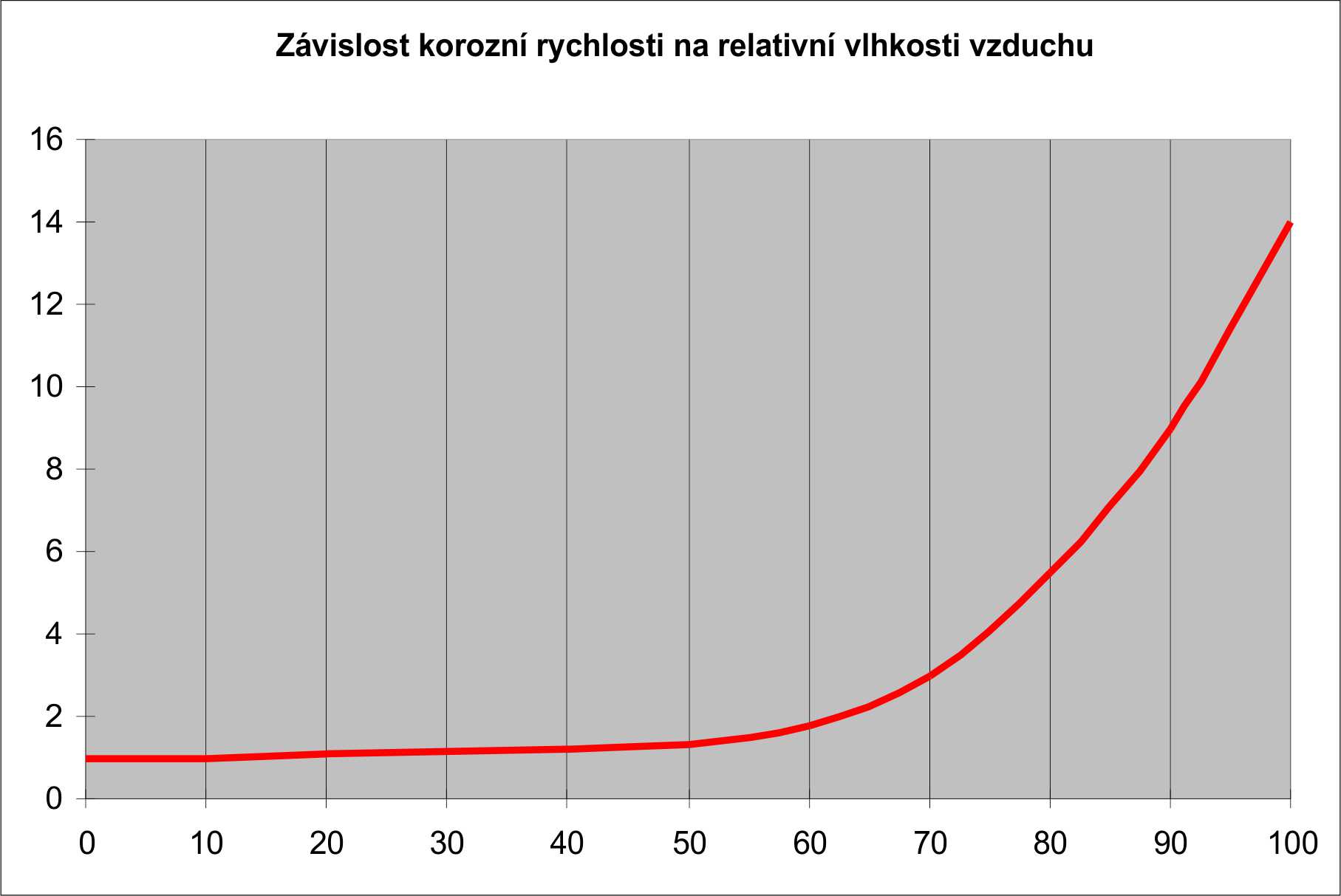
It can be seen from this graph that if the relative humidity of the air is up to 65%, the corrosion rates are low. Above 65%, however, speeds rise steeply. At 100%, i.e. in liquid water, corrosion rates are highest.
But corrosion rates are also dependent on temperature. They grow exponentially with higher temperature.
But they also depend on which chemical compound they come into contact with during the manufacturing process. And the issue of pH is related to this. In acidic environments, corrosion rates are high and the curve begins to break at pH 7.5. Above pH 8.5 they are relatively small.
A number of important general practical conclusions follow from the above. Steels (with the exception of stainless steel) will always corrode without treatment. How quickly, and on what corrosion products, it depends on many factors.
There is no machining process that can remove or stop corrosion. None of them can work with electrons. So, even if the surface is beautifully shiny and without visible corrosion, the electrochemical corrosion processes in the material continue and it is only a matter of time before the corrosion reappears. It is also necessary to keep in mind that it can appear up to 30 cm from the place of its origin. Corrosion can be stopped or prevented only by chemical processes, to which some metallurgical ones belong as a smaller group.
Some alloying additives, or elements present from the manufacturing process, accelerate corrosion processes. And their quantity also plays an important role. Even if it is the same grade of steel and from the same manufacturer, each batch of melting and processing will have slightly different corrosion behavior. Sometimes even significantly different.
The formation of micro-galvanic cells can be reduced by not allowing dust from the air to settle on the steel product or raw material. But it is also necessary to pay attention to process fluids, such as machining emulsions, oils, quenching fluids, welding agents, etc. Over time, these become clogged with micro-dust from machining, which creates ions. And filters that could remove them are not used in the metal industry. Therefore, it is absolutely necessary to replace the given fluids with clean ones from time to time.
Related to this is the issue that these liquids usually react with air. New compounds are often formed, which sometimes initiate the formation of corrosion. But they also get microbial contamination from the surrounding environment. And many microorganisms produce metabolites that are highly corrosive. This results in the need not only to continuously change process fluids, and to find the limit of their endurance in the process before problems start to arise, but also to clean and disinfect the equipment before a new process fluid is fed into it.
And if steel and then copper or aluminum alloys are processed in the same production process, it can be called a nice mess. In such a case, reliance is placed on the fact that micro galvanic cells are created. And since chemicals that could stabilize such combinations are produced in small quantities and only for a few technological operations, it is better to machine and treat steels separately from copper alloys and aluminum alloys.
Industrial washing and degreasing is "one big fun" that deserves a separate treatise.
Another important factor affecting the corrosion of steel is the contact of the products with each other. The formation of crevice corrosion occurs just because, for example, sheets are stacked on top of each other during storage. Or when the products are packed in such a way that one touches the other. One company wanted to save on shipping costs, so they stacked the products as close as possible to each other in the shipping packaging, and they couldn't help but wonder how they started getting complaints. In such cases, it is necessary to use some kind of dielectric so that the products do not touch directly, or to make some suitable anti-corrosion protection before they come into contact. Another example of contact corrosion is fig.

Surprisingly, few people know that the differently treated surface of the same product has a great influence on the corrosion behavior. For example, one company used high machining speeds to create "notches" in one spot on the product, while the rest of the workpiece was smooth. The place corroded very quickly, even though the entire product was preserved. A suitable solution is for the entire product to have the same surface roughness as possible, or an effective conservation procedure needs to be found.
It is also neglected that the very shape of the product has an effect on the formation of corrosion and its speed. Sharp corners and L-shapes support this cause of corrosion. Another company prevented the development of corrosion by starting to machine a rounded inner corner instead of an almost sharp right angle.
Relative humidity alone provides big corrosion surprises. It is quite often forgotten that even if in production there is 50% relative humidity at 20°C, at a temperature of 11°C this air reaches 100% relative humidity. Then the companies are surprised that their products corroded during transport, when they are fine in the warehouse directly at the factory. It's just that they forgot about vapor condensation due to the reduced temperature during transport.
And there is one very important matter related to that. Many companies pack their products in PE films. Some of them even fuse smaller products into them. However, the less permeable the packaging is to water vapor, the harder it breathes, the more moisture condensation occurs inside. Basically, in this way, a person creates a condensation chamber, which accelerates corrosion. And even though there are foil materials that contain vapor corrosion inhibitors, their use is very risky. No film contains enough inhibitor to be effective more than a few mm from its surface. Most products cannot be wrapped in such a way that their entire surface is in contact with such an anti-corrosion film. The idea is to pack in paper laminates impregnated with a vapor corrosion inhibitor, but in such a way that the packaging has enough slits through which it can breathe and that there is as little condensation of water vapor as possible. It is also a harmful habit to put a bag with silica gel in the foil package and then seal the package hermetically. But no one dries the silica gel just before putting it in the package, so by the time the factory receives the shipment of silica gel bags, they are already unusable because they are saturated. And with that, the user only adds additional moisture to the moisture contained in the air in the package, which is retained by the silica gel.
Please do not underestimate the effect of temperature on corrosion. Every 10°C, the rates of chemical reactions double. This means that if someone processes at 20°C and processes the exact same material at 80°C, the reactions will be 64 times more intense and faster at that 80°C.
When machined under emulsion, it is an electrically conductive environment with high corrosion rates. Therefore, it is necessary that emulsions contain anti-corrosion additives. But there is a catch in that too. The entire production process and the means used in it must be compatible with each other. Or it can easily happen that one anti-corrosion additive reacts with another to create spectacular corrosion. If the manufacturer is not sure of the composition of the process fluids and what they will do to each other, it is a good idea to consult.
For example, combining contact corrosion inhibitors in oil systems with vapor corrosion inhibitors is a nonsense that will come pretty expensive.
But many manufacturers also believe that if they machine under oil, nothing can happen. The opposite is true. Mineral oils contain 4-8% water, which is more than enough to cause corrosion. With synthetic oils, the situation is even more complicated. Some of them do not accept water at all and it then accumulates somewhere, or some of them are infinitely soluble in water.
In the next installment, I will focus on the most common production corrosion problems and how to deal with them.
Ing. Peter Stuchlík, CSc., CTex ATI