Blog
Anti-corrosion protection using vapor corrosion inhibitors
Anti-corrosion protection using vapor corrosion inhibitors
Anti-corrosion protection using vapor corrosion inhibitors
Ing. Peter Stuchlík, CSc., CTex ATI
Motto: "It is cheaper and faster to prevent problems than to solve them when they arise."
Although corrosion degradation processes concern a whole range of materials, further attention will be paid only to corrosion processes of metals, especially iron alloys.
Once the corrosion reaction is started, it is very difficult and expensive to stop it because electrons or ions are transferred through the crystal lattice of metals. Therefore, it is important to always keep in mind that potential accumulation and corrosion processes can occur relatively far from the place where the corrosion started (for iron alloys, the usual distance is 30 cm). And it is delusional to think that there is a mechanical process that can stop corrosion. Grinding does not remove one particular electron.
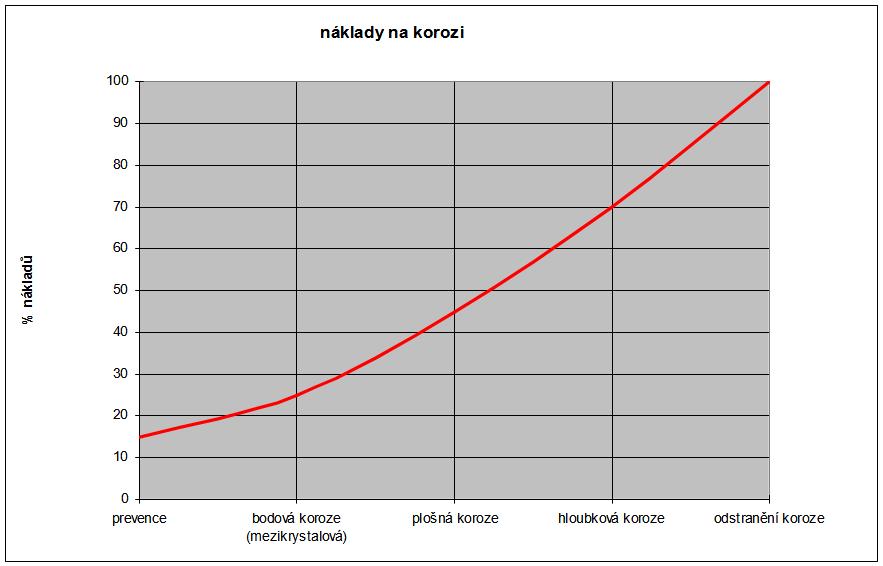
The following figure roughly shows the dependence of the costs of stopping and removing corrosion.
Giant. no. 1
Dependence of the costs of stopping the corrosion of steels according to their type.
According to the method of attack, corrosion is divided into uniform, where corrosion processes take place on the entire surface at the same speed and to the same depth. Further to uneven, which can be dimple, point, intercrystalline, transcrystalline, lamellar and selective.
According to the physical process, we distinguish corrosion under voltage, thermal corrosion, cavitation, corrosion by stray currents, corrosion by induced voltage, form corrosion, etc.
According to the chemical process, it is divided into corrosion in an electrically non-conductive environment, which can be in reducing gases, in oxidizing gases and in non-conductive liquids. Or for corrosion in a conductive environment (electrochemical corrosion), when electrochemical, electrolytic or concentration cells are formed (concentration cells include joint or crevice corrosion).
Special types of corrosion include microbial or high-energy radiation.
According to the phase interface, it is divided into solid phase (metal) with gas, solid phase/liquid, solid phase/solid phase (contact corrosion).
In practice, we most often encounter two types of corrosion in metals:
Chemical corrosion in an electrically non-conductive environment. That is, most often a reaction between a metal and a reducing or oxidizing gas at the phase interface. These are mostly reactions with oxidizing gases. But the case of hydrogen depolarization using (H + ), i.e. corrosion in reducing gases, is also quite common. In the case of iron alloys, this type of corrosion has a significant rate only when the temperature exceeds 580°C.
However, the most common and also the most dangerous are cases of corrosion in an electrically conductive environment, i.e. electrochemical corrosion. Corrosion velocities here are ten to a thousand times higher than in a non-conductive environment, and fractions of seconds are enough to initiate corrosion processes. The critical value for the development of these processes is 60% relative humidity. Once this value is exceeded, corrosion rates increase exponentially.
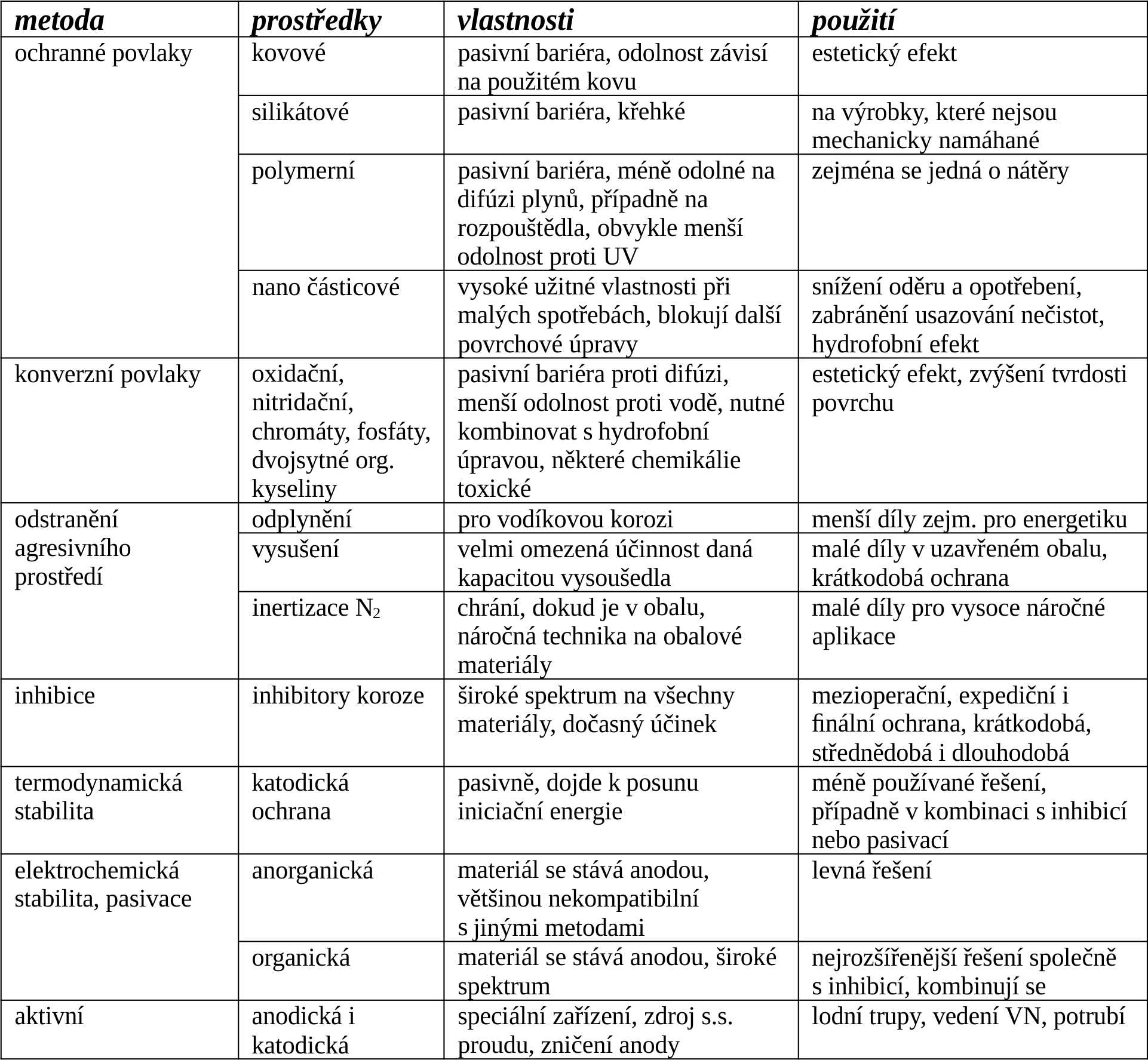
A number of basic methods of corrosion protection have been discovered and others are being worked on. In practice, it is not possible to use one corrosion protection system for all types of corrosion processes, materials and metal products. In the same way, it is necessary to find an optimal compromise between economic, useful, ecological, health and technological factors.
The basic methods of protecting metals against corrosion include:
-
Increasing the chemical inertness of the surface using protective coatings (passive protection). It is a surface blocking that prevents contact of aggressive chemicals with the metal surface and prevents the diffusion of gases. This is done using metals - either alloying, or barrier plating, or cathodic plating. Using non-metals - oxidized, nitrided, chromated and phosphated conversion coatings. Using silicates - enamels and glass. Using polymers – paints, varnishes, polymer melt coating. Or hydrophobic treatment using "waxes", oils, etc. is used. The latest is blocking using nanoparticles, which can be metallic or non-metallic.
-
Modification of the corrosion environment. By removing the aggressive component (degassing, drying, inertization).
-
Change of environment (inhibition). Physical inhibition is used - blocking of active nuclei on the surface, or chemical inhibition of the surface. It can be anodic or cathodic.
-
Increasing the thermodynamic stability of the surface (cathodic protection).
-
Increasing the electrochemical stability of the surface by shifting the reaction balance (anodic protection or passivation).
-
Active protection using a sacrificial electrode (anodic protection) or active cathodic protection.
A good anti-corrosion treatment technology usually combines several principles at the same time. Most often, it is a combination of protection using a passive or hydrophobic layer, using inhibition and increasing the electrochemical stability of the surface.
The basic methods of anti-corrosion protection and their properties are listed in the following table:
When designing the most suitable technology and method of anti-corrosion protection, it is necessary to take into account:
-
What kind of metal is it, and which risky additives does it contain.
-
What technology was used to make the product?
-
What pollution occurs on the surface of the product.
-
What method of anti-corrosion protection is used by the manufacturer in individual production steps, or what is required by the customer. Here, the compatibility of the protective means used, both with each other and with the contamination on the product, is very important.
-
How the product will be stored and transported. And under what conditions. What protection period is required.
-
How the product will be handled in the future. (For example, it is not possible to use a difficult-to-remove anti-corrosion protection that completely blocks the surface of the product, if further surface treatment is to be carried out on it.)
Since corrosion prevention is the cheapest anti-corrosion measure, it is important to pay extra attention to it. Several principles apply to prevention:
-
The product must not be exposed to relative humidity above 60% . This cannot be avoided in a number of processes, because they are machined under aqueous emulsions, water quenching and cooling or pickling baths are used, products are washed in aqueous media, etc. In these cases, it is imperative to ensure that the products are dried as quickly as possible. The same is true of condensed moisture or rain.
-
If corrosion is detected during any manufacturing step, the necessary corrective measures must be taken immediately . Once the corrosion reaction is started, it will not stop on its own even if the corrosive conditions are removed. It is absolutely necessary to shift the reaction balance by means of some anti-corrosion system (passivation, inhibition).
-
Pay attention to the compatibility of the means used in the entire production process from start to finish. This is especially true if different machining and process fluids are used. They represent a rather complex chemical system that contains a number of additives. Therefore, it is imperative to carry out appropriate corrosion tests for compatibility.
-
If any corrosion protection methods and means are used in the manufacturing process, they should be maintained throughout the process. In the event that for some reason the combination of different resources cannot be avoided, it is usually necessary to include an operation that completely removes the previous resources. Here too, it is absolutely necessary to carry out suitable corrosion tests.
-
For iron alloys, all operations with increased humidity or in the presence of an electrolyte (water) should take place in conditions above pH 7.5 (optimally in the range of pH 8-10). If any operation takes place in an acidic region (i.e. below pH 7), neutralization should be included immediately and a check made for both corrosion and the remains of the neutralization reaction.
-
For most metals, it is desirable that the product does not come into contact with chlorides, chlorine derivatives, sulfur dioxide (SO 2 ) or compounds that release it, and with most monoacids. The corrosion reactions started by them are difficult to stop.
-
Make sure that the contact of two surfaces in the presence of water vapor or water does not create an electrochemical (galvanic) cell . This occurs when two metals with different compositions are in contact (even different grades of steel are enough) or when two differently treated surfaces come into contact. In such cases, it is necessary to separate the surfaces from each other dielectrically, ensure a reduction of humidity below 60% relative humidity, prevent water condensation and take anti-corrosion measures.
-
Limit the formation of electrolytic or concentration cells by means of shape and design solutions. Choose rounded corners over sharp ones and prevent the formation of capillaries. If this cannot be prevented for some reason, then it is necessary to ensure that the humidity is reduced below 60% relative humidity, prevent water condensation and take anti-corrosion measures.
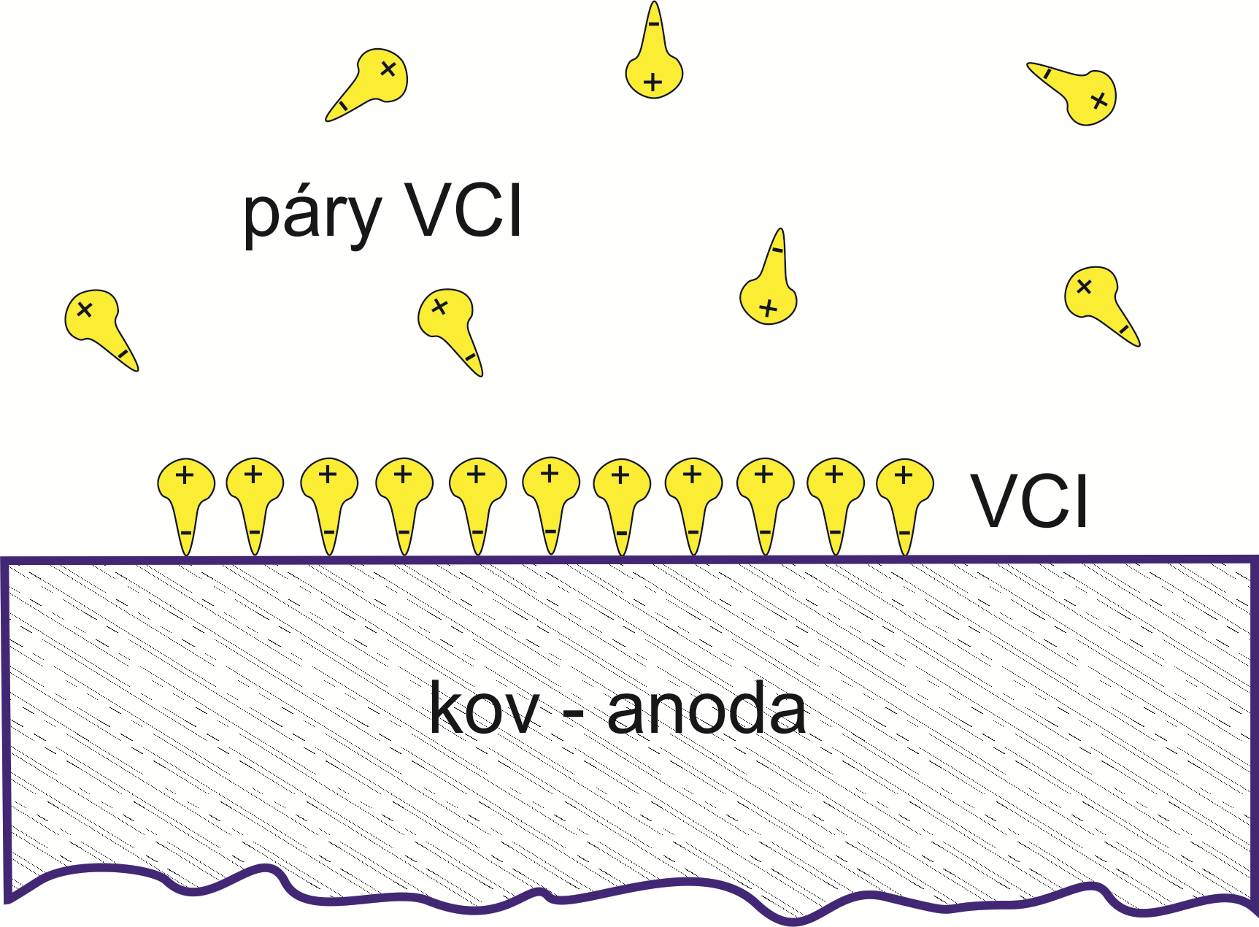
One of the ways to prevent corrosion is the use of vapor corrosion inhibitors (VCI). These inhibitors are evaporated from a carrier under normal pressure and temperature, the molecules of the inhibitor are attached to the surface of the metal by physical or chemical bonds, where they form a molecular layer. This layer usually has a pressure greater than the partial pressure of water vapor, so they have a hydrophobic effect. They therefore have the function of physical inhibition. At the same time, they also function as cathodic or anodic chemical inhibition. Some of them are also capable of passivation reactions during the formation of conversion layers. Other of them can also neutralize corrosive ions, or function as buffers shifting the pH to the alkaline region, or as scavengers of free radicals. The diagram of the action of vapor corrosion inhibitors is shown in the following figure.
Giant. No. 2
A method of attaching VCI vapors to a metal surface
There are a number of vapor corrosion inhibitors and they are divided into several groups.
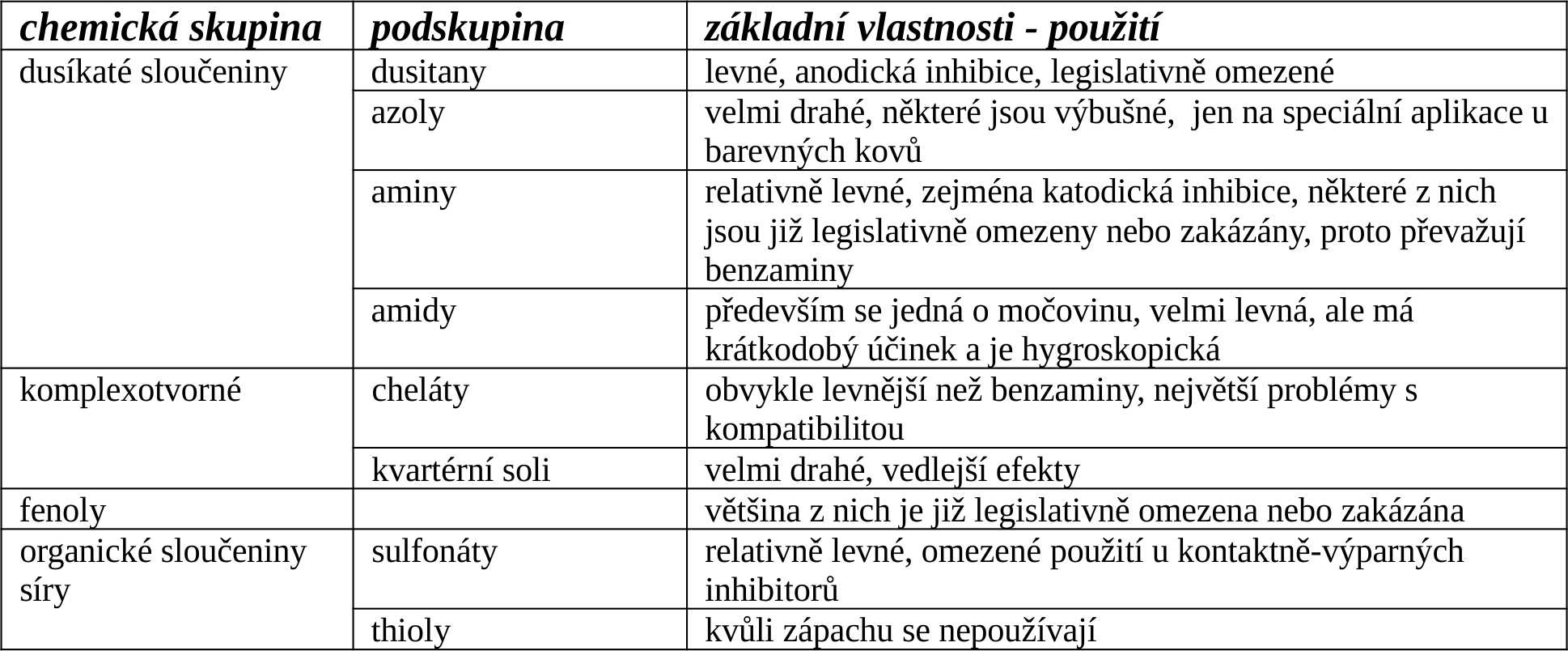
Currently, amines are the most widely used vapor corrosion inhibitors in the world, followed by chelates, nitrites (although the last two types are on the decline due to legislative restrictions) and urea (for short-term protection).
Advantages and disadvantages of vapor corrosion inhibitors:
Advantages:
-
wide range of applications and applicability to all metals,
-
high ratio of effectiveness to the price and the amount of inhibitor used,
-
the surface of the products is not greasy and it is usually not necessary to remove the inhibitor before further surface treatment,
-
simple application.
Disadvantages:
-
must be used in closed or semi-closed containers,
-
they are mostly water soluble, so rain or condensed moisture will break them down,
-
are sensitive to the pH of the environment,
-
have limited thermal stability,
-
it takes some time for the vapors to reach an effective concentration.
All inhibitors of this group are very similar and have some common features. Their thermal decomposition temperature is about 70° C, the "start" temperature is about 15° C, they are soluble in water, they provide a high efficiency-to-mass ratio, the protective effect is about 1 year for packaged goods stored in a tempered warehouse (if they are used correctly ), a certain concentration of vapors must be reached in the package to be effective and they reach 80-95% efficiency in protecting roughly 4 dm 3 of the interior space of the package per 1 g of inhibitor. See Chart.
Giant. No. 3
Anti-corrosion efficiency of the amount of VCI used depending on the volume of the package to be protected [g/dm 3 ].
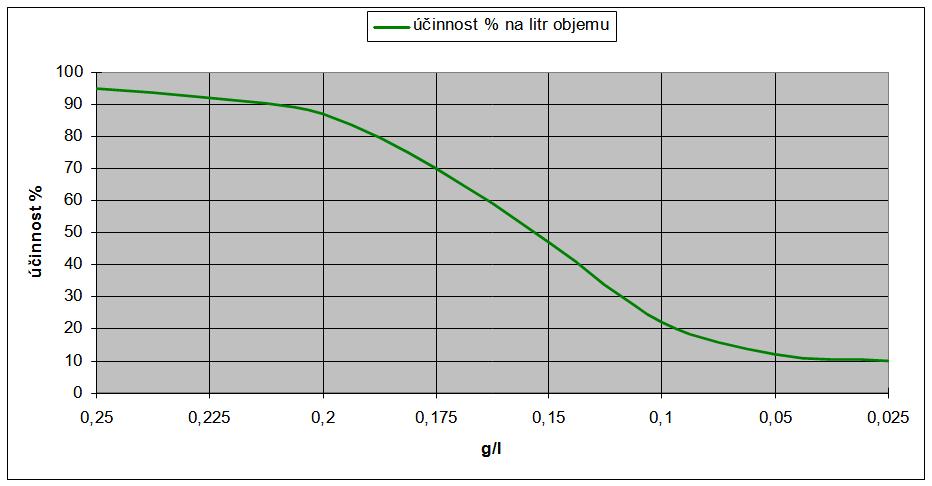
Specific types of inhibitors from individual manufacturers differ from each other within a certain range in terms of solubility, surface tension, vapor pressure, protected metals, percentage of anti-corrosion efficiency, health safety, biological degradability, or other details.
Benzamine corrosion inhibitors are solid substances that usually have the character of micro crystals. It is possible to sprinkle them directly on the protected goods, but this method is used only exceptionally. It is more common to find that inhibitors are filled into bags, or most often a carrier is filled with them.
The most common carrier is carbonized VCI papers. These are produced both smooth and creped, without lamination, or laminated with PE film, or with textile reinforcement.
PE films filled with VCI are currently a popular carrier. Such foils are used by those who know little about the issue. By adding VCI to the polymer, the mechanical properties of the film deteriorate. An acceptable value of fulfillment is between 2-4%. While most packaging films have an area weight of up to 100 g/m 2 . Therefore, the packaging film contains a very small amount of the active substance relative to its surface area. Moreover, the release of the inhibitor is slowed down by the necessity of diffusion of VCI through PE, and the specific surface area of the foil is on the order of thousands of times smaller than that of paper. Therefore, the use of foils for anti-corrosion protection is very illusory.
VCI are also filled into PE granulate and sprinkled with packaged goods. This method of protection has all the disadvantages of a film, as well as the disadvantage of being removed as a powder, but without its effectiveness.
Furthermore, various sponges, felts, non-woven fabrics or bulky papers are used as carriers. These carriers are cut into blanks and then inserted into the protected area.
Since there is a wide range of VCIs, attention will be paid only to general knowledge and a little more detailed information will be focused on benzamine VCIs.
With regard to chemical substances, the most important legal measure is the REACH-CLP regulation. But there are other rules and regulations. Lists of dangerous substances are attached to these legislative measures. It is therefore necessary to check that the VCI in question does not appear on the listed lists and, if so, which dangerous category is assigned to it. (A number of historical VCIs are on this list in terms of carcinogenicity, or suspected carcinogenicity.) Moreover, the risks of a specific agent should be expressed in its Safety Data Sheet.
Current commercial quality benzamine inhibitors are only irritants, and this is proven by LD50 measurements, and they are biodegradable. Furthermore, they do not contain secondary amines, N-nitroamines or their precursors. Some corrosion inhibitors that were used in the past were more effective, but their properties are now unacceptable from a health or environmental point of view.
Regardless of how vaporous corrosion inhibitors (VCI) are applied, product packaging plays a very important role. Several principles apply. The products must not be packed wet , because they would be exposed to conditions of increased humidity, such as in a condensation chamber, at the very moment when they are most vulnerable (before the protective atmosphere is created). Such an environment would very quickly nullify the inhibitor's protective effect. At the same time, the products must not be packed corroded , because VCI do not have the ability to remove corrosion and are able to stop corrosion as much as possible, but at the cost of their consumption.
One of the most common mistakes when preserving metal products with VCI is to first preserve the product with oil and then place it in a VCI vapor atmosphere for "better" protection. Most of the time it turns out the opposite, i.e. badly.
Even though VCI vapors are able to penetrate remote spaces and very small capillaries, it should be taken into account that the VCI source should not be further than 30 cm from the protected surface.
In order to achieve the desired concentration of VCI vapors, it is necessary to close the product. Either completely closed or semi-closed packages are used. In the case of closed packages, care must be taken to prevent moisture condensation inside the package during storage or transport.
Semi-closed packaging allows air ventilation between the external environment and the internal contents. At the same time, they are a sufficient barrier to create the necessary concentration of VCI vapors.
When packaging products, it is also necessary to take into account corrosion, which is caused by the contact of two metals or two unequally treated surfaces in an electrically conductive environment. It is absolutely necessary to separate these surfaces from each other dielectrically . For example, the product must not touch the walls of the metal box. Dielectric separation can be achieved using VCI carbonated paper or laminated paper. The foils themselves may not be so suitable, because in the case of moisture condensation, a concentration cell can form between them and the metal (pitting corrosion).
Last but not least, it is necessary to pay attention to how the products are stored in group packaging. In the case of packaging, it cannot be completely ruled out that condensation of atmospheric moisture will occur. This moisture then flows down due to gravity to the lowest places, or is held in the crevices due to capillary forces. Removing capillary water is an energy-intensive and lengthy process. It is therefore necessary to solve the storage of the material in the package in such a way that possible condensed water does not penetrate into the cracks and capillaries, and that this water is removed as quickly as possible from the places where it flows.